Search results
Search for "halogen bonding" in Full Text gives 25 result(s) in Beilstein Journal of Organic Chemistry.
Green and sustainable approaches for the Friedel–Crafts reaction between aldehydes and indoles
Beilstein J. Org. Chem. 2024, 20, 379–426, doi:10.3762/bjoc.20.36
- . Finally, another nucleophilic attack by a second molecule of indole to IV is occurring, forming the desired BIM 12, while simultaneously releasing the catalyst, rendering it available for another catalytic cycle [80]. Halogen bonding processes Recently, halogen bonding (XB) interactions have emerged as an
- be addressed. The most recent application of halogen bonding in the synthesis of BIMs was introduced in 2023 by Galathri et al., who employed an N-heterocyclic iod(az)olium salt as the monodentate catalyst [96]. This approach utilized water as the reaction solvent and employed a low catalyst loading
- of just 0.5 mol %, while providing satisfying yields (60–96%) in just 1 hour. The employment of a green aqueous medium, the mild reaction conditions and the relatively broad substrate scope are some of the benefits that render this protocol more efficient than previous halogen-bonding methodologies
Exploring the role of halogen bonding in iodonium ylides: insights into unexpected reactivity and reaction control
Beilstein J. Org. Chem. 2023, 19, 1171–1190, doi:10.3762/bjoc.19.86

- Carlee A. Montgomery Graham K. Murphy Department of Chemistry, University of Waterloo, 200 University Ave W., Waterloo, Ontario, N2L3G1, Canada 10.3762/bjoc.19.86 Abstract Halogen bonding is commonly found with iodine-containing molecules, and it arises when Lewis bases interact with iodine’s σ
- -holes. Halogen bonding and σ-holes have been encountered in numerous monovalent and hypervalent iodine-containing compounds, and in 2022 σ-holes were computationally confirmed and quantified in the iodonium ylide subset of hypervalent iodine compounds. In light of this new discovery, this article
- provides an overview of the reactions of iodonium ylides in which halogen bonding has been invoked. Herein, we summarize key discoveries and mechanistic proposals from the early iodonium ylide literature that invoked halogen bonding-type mechanisms, as well as recent reports of reactions between iodonium
Direct C2–H alkylation of indoles driven by the photochemical activity of halogen-bonded complexes
Beilstein J. Org. Chem. 2023, 19, 575–581, doi:10.3762/bjoc.19.42
- deuterated acetonitrile (Figure 3). Interestingly, a change in chemical shift of the diagnostic α-protons of 2a was displayed upon addition of increasing amounts of DABCO, suggesting the presence of the halogen-bonding interaction [30]. More precisely, the 1H NMR signal of the α-hydrogens (Hα) within 2a was
- found to shift to lower ppm values because the Hα nuclei have been affected by higher electron density caused by the formation of the halogen-bonded complex between 2a and DABCO. To confirm that the shift of Hα was indeed produced by a halogen-bonding interaction, 19F NMR analysis of compound 2d, which
- absorption spectra recorded in acetonitrile in 1 cm path quartz cuvettes. [DABCO]: 0.5 M; [2a]: 0.5 M. 1H NMR titration of DABCO in a solution of 2a in ACN-d3 to detect their halogen-bonding association through the shift of the signal of Hα. Proposed reaction mechanism for the photochemical alkylation of 1a
Synthesis and reactivity of azole-based iodazinium salts
Beilstein J. Org. Chem. 2023, 19, 317–324, doi:10.3762/bjoc.19.27
- observed a coordination of the triflates along the C–I axis with distances of 2.705 Å (I1–O1) and 2.898 Å (I1–O5). For the ortho-methyl-substituted analogue 5ax, no halogen bonding to the triflates was observed, indicating an effective steric protection of the σ-holes [36]. Instead, there were only two
- result obtained when using the stabilized salt 12 (Scheme 2b) [44]. Here, no counter-ion exchange to chloride was observed. The favored counter ion is determined by the pKa value of the corresponding acids but not by halogen bonding due to the steric hindrance at the iodines’ σ-holes. The reaction of
Supramolecular approaches to mediate chemical reactivity
Beilstein J. Org. Chem. 2022, 18, 1463–1465, doi:10.3762/bjoc.18.152

- hydrogen bonding interactions play a pivotal role in catalysis. More recently, halogen bonding interactions have been used as a novel tool to catalyze a wide variety of processes. Other nonclassical interactions, including anion-, chalcogen-, and pnictogen bonding, have also been exploited for the design
BINOL as a chiral element in mechanically interlocked molecules
Beilstein J. Org. Chem. 2022, 18, 508–523, doi:10.3762/bjoc.18.53

- to slightly better stereodiscrimation of the guest molecules (see Figure 17). Subsequently, Beer and co-workers reported the first example of a chiral halogen-bonding [3]rotaxane for the recognition and sensing of dicarboxylate anions [64]. The [3]rotaxane (S)-68 was prepared in a two-fold clipping
A Se···O bonding catalysis approach to the synthesis of calix[4]pyrroles
Beilstein J. Org. Chem. 2022, 18, 325–330, doi:10.3762/bjoc.18.36
- noncovalent forces, hydrogen bonding interactions play a central role in noncovalent catalysis [2] while halogen bonding interactions have lately been exploited as a new tool to catalyze a diverse array of reactions [3][4][5]. In addition, nonclassical interactions such as anion–π [6][7][8][9][10][11] as well
Structural effects of meso-halogenation on porphyrins
Beilstein J. Org. Chem. 2021, 17, 1149–1170, doi:10.3762/bjoc.17.88

- architecture based on C–I···N and C–I···π interactions was formed through halogen bonding in the lattice of self-assembly of meso-tetraarylporphyrins [7]. In recent years there has been a strong uprising interest for substituting hydrogen-bonding motifs with their halogen-bonding counterparts. This is due to
- pointing towards the phenyl rings in a ↑↓↑↓ repeating pattern (Figure 2C). However, in this structure, there is no evidence for halogen bonding. By the addition of a nickel(II) metal center to compound 1, we obtain the structure of compound 2 [27]. In this structure a variety of changes in the overall
Tuning the solid-state emission of liquid crystalline nitro-cyanostilbene by halogen bonding
Beilstein J. Org. Chem. 2021, 17, 124–131, doi:10.3762/bjoc.17.13

- tuning of the fluorescence behaviour and mesomorphic properties of the assemblies. Keywords: fluorescence; halogen bonding; liquid crystal; Introduction Supramolecular chemistry has proven to be an efficient approach for the development of novel smart materials, since it relies on non-covalent
- supramolecular liquid crystals, especially hydrogen bonding and halogen bonding have gained considerable attention [3][4][5][6][7]. In 2004, Bruce and co-workers reported the first example of a halogen-bonded liquid crystal based on pentafluoroiodobenzene and 4-alkoxystilbazole [5]. Ever since, several other
- groups employed halogen bonding for the formation of liquid crystalline materials [8][9]. For instance, Palacio et al. used (E)-1-(4-(octyloxy)phenyl)-2-(2,3,5,6-tetrafluoro-4-iodophenyl)diazene as a photo-switchable halogen bond donor and investigated the light-induced phase transition of the complexes
Clickable azide-functionalized bromoarylaldehydes – synthesis and photophysical characterization
Beilstein J. Org. Chem. 2020, 16, 1683–1692, doi:10.3762/bjoc.16.139
- ISC processes through intermolecular halogen bonding to generate efficient RTP was initially investigated by Kim et al. [58]. They developed the minimalistic 2,5-dihexyloxy-4-bromobenzaldehyde (1) [59][60][61][62][63] which showed a weak fluorescence in solution, but exhibited a green phosphorescence
Models of necessity
Beilstein J. Org. Chem. 2020, 16, 1649–1661, doi:10.3762/bjoc.16.137
- approach leads to unnecessary complication of the model. As these interactions together with hydrogen and halogen bonding can all be treated within the single “σ-hole” framework [92], a single unified approach seems possible. However, such an approach would require a wave function or electron density
- interaction between halogens and nucleophiles as repulsive; whereas we now know that halogen-bonding attractions can be as strong as hydrogen bonds. There will be more such examples but it is important to identify the encompassing phenomenon, rather than defining a wealth of apparently unique interactions
Development of fluorinated benzils and bisbenzils as room-temperature phosphorescent molecules
Beilstein J. Org. Chem. 2020, 16, 1154–1162, doi:10.3762/bjoc.16.102
- restriction of intramolecular motions [23]. Moreover, crystalline 2,5-dihexyloxy-4-bromobenzaldehyde displays green phosphorescence, which stems from rapid ISC due to the heavy atom effect via halogen bonding (C=O···Br) [24]. Moreover, benzophenone- or benzil-type molecules can achieve long-lived
Halogen-bonding-induced diverse aggregation of 4,5-diiodo-1,2,3-triazolium salts with different anions
Beilstein J. Org. Chem. 2020, 16, 78–87, doi:10.3762/bjoc.16.10
- triazolium salts show diverse aggregation via halogen bonding between C–I bonds and anions. Triazolium with halide anions exists as a tetramer with saddle conformation. Triazolium tetrafluoroborate exists as a trimer with Chinese lantern shape conformation. Triazolium trifluoroacetate and acetate exist as
- the former shows a boat conformation and the latter forms a rectangle conformation. Triazolium salts form a linear polymer with polyiodide. 2-BF4 forms co-crystals with 4,4'-bipyridine via halogen bonding. DFT calculation results show that the σ holes of 4,5-diiodo-1,2,3-triazolium is similar to the σ
Fluorinated azobenzenes as supramolecular halogen-bonding building blocks
Beilstein J. Org. Chem. 2019, 15, 2013–2019, doi:10.3762/bjoc.15.197
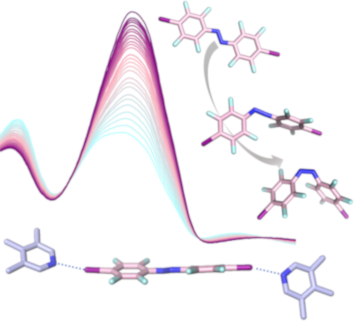
- building blocks both in solution and in the solid state in combination with neutral halogen bonding acceptors, such as lutidines. Therefore, we investigate the photochemistry of a series of azobenzene photoswitches. Upon introduction of iodoethynyl groups, the halogen bonding donor properties are
- azobenzenes with different halogen bonding donor properties are discussed in relation to their changing photophysical properties, rationalized by DFT calculations. Keywords: azobenzene; DFT calculations; fluorine chemistry; halogen bonding; photochemistry; Introduction The halogen bond is an attractive
- noncovalent interaction between a polarized halogen atom (the halogen bond donor) and a Lewis base (the halogen bond acceptor) [1][2]. A prominent example regarding the origin of halogen bonding can be found in inorganic solid-state chemistry. The structurally diverse group of polyiodides, with its rich
Halogen bonding and host–guest chemistry between N-alkylammonium resorcinarene halides, diiodoperfluorobutane and neutral guests
Beilstein J. Org. Chem. 2019, 15, 947–954, doi:10.3762/bjoc.15.91

- guest inclusion in solution. Keywords: capsule; dimeric assemblies; halogen bonding; host–guest chemistry; resorcinarene salts; X-ray crystallography; Introduction The construction of specific supramolecular assemblies based on the directional non-covalent bonding has been a central goal of
- supramolecular chemistry and materials science [1][2][3]. New systems both help us to better understand the nature and impetus behind the self-assembly of these fascinating systems, while also providing new materials that can provide the basis for a wide number of applications [4][5]. Halogen bonding (XB), as a
- crystallography: MeOH-MeCN@1&DIOFB and Water@2&DIOFB. These two structures, besides illustrating the potential of halogen bonding for organizing complex capsular systems, shed light on the importance of flexibility in affecting the self-assembled systems. Results and Discussion Single crystal X-ray diffraction
Mechanochemistry of supramolecules
Beilstein J. Org. Chem. 2019, 15, 881–900, doi:10.3762/bjoc.15.86
- chemistry reactions, as it relies on soft force [97][98] or non-covalent interactions [2] such as hydrogen bonding [99], cation–π [100][101][102], anion–π [103], hydrophobic effect [104][105], halogen bonding [106][107][108][109], etc. As enzymes are structurally complex entities and are difficult to modify
- has been shown to proceed with excellent turnover numbers. Recently, Friščić and Cinčić with co-workers reported an elaborative study on the halogen bonding between 1,3,5-trifluoro-2,4,6-triiodobenzene and triphenylphosphine, -arsine, and -stibine under neat mechanochemical conditions or through
Pyrene–nucleobase conjugates: synthesis, oligonucleotide binding and confocal bioimaging studies
Beilstein J. Org. Chem. 2017, 13, 2521–2534, doi:10.3762/bjoc.13.249

- distance = 2.882(2) Å) and halogen bonding (C30–Cl31···O28 distance = 2.972(2) Å) observed in the crystal packing of 2. UV–vis absorption and fluorescence spectra of pyrene–adenines 5 (a) and 3 (b) in diluted (c ≈ 10−5 M) dichloromethane solutions at ambient temperature. Absorption changes during titration
Solvent-free copper-catalyzed click chemistry for the synthesis of N-heterocyclic hybrids based on quinoline and 1,2,3-triazole
Beilstein J. Org. Chem. 2017, 13, 2352–2363, doi:10.3762/bjoc.13.232
- halogen bonding observed in their crystal structures. Milling procedures using Cu(II), Cu(I) and Cu(0) catalysts proved to be significantly more efficient than the corresponding solution reactions, with up to 15-fold gain in yield. Both procedures showed the same reactivity trend, resulting in the H < Cl
New approaches to organocatalysis based on C–H and C–X bonding for electrophilic substrate activation
Beilstein J. Org. Chem. 2016, 12, 2834–2848, doi:10.3762/bjoc.12.283
- hydrogen bonds or halogen bonding (C–X···X or C–X···A interactions) in organocatalyst design. Such non-covalent interactions have been traditionally viewed as “weak” when compared to classical A–H···A hydrogen bonds. However, in some cases the term “weak” may be misleading as an increasing number of
- examples demonstrate the effectiveness of such interactions for organocatalyst design. While C–H···A hydrogen bonds have been invoked in biological processes, halogen bonding is not commonly observed in natural enzyme-catalyzed reactions. Therefore, application of these new interactions for small molecule
- bond was found to be ≈180°. Since these reports, numerous other examples of halogen bonding have been uncovered, and halogen bonding has become of great importance to the fields of chemistry and materials science. Not only halogens, but also neutral halogen-containing organic molecules were found to
My maize and blue brick road to physical organic chemistry in materials
Beilstein J. Org. Chem. 2016, 12, 229–238, doi:10.3762/bjoc.12.24

- structures, such as ribbons, fibers, and sheets. This self-aggregation is driven by noncovalent interactions, including hydrogen bonding, π stacking, van der Waals interactions, and halogen bonding. Physical interactions amongst these larger structures lead to gel formation. Because noncovalent interactions
Supramolecular chemistry: from aromatic foldamers to solution-phase supramolecular organic frameworks
Beilstein J. Org. Chem. 2015, 11, 2057–2071, doi:10.3762/bjoc.11.222

- mixtures, although I had been to Beijing for X-ray diffraction experiments at Peking University. We thus proposed that the two compounds formed 1:1 charge-transfer complexes (Scheme 1). Currently, this N···I interaction is termed as halogen bonding, which is widely used in supramolecular crystal
- molecules to evaluate their binding to these triazole foldamers. He finally found that these foldamers were good halogen bonding receptors for tritopic and ditopic guests, including 30 in dichloromethane or its mixture with hydrocarbons. Yanhua further utilized the C–H····F hydrogen bonding to induce the
Trifluoromethyl-substituted tetrathiafulvalenes
Beilstein J. Org. Chem. 2015, 11, 647–658, doi:10.3762/bjoc.11.73
- of secondary non-bonding interactions such as hydrogen or halogen bonding [6][7], an issue of current strong interest in organic solid state chemistry [8][9]. However, it was also recognized that the introduction of only one or two of such EWG on the TTF core could limit this anodic shift, and
Computational evidence for intramolecular hydrogen bonding and nonbonding X···O interactions in 2'-haloflavonols
Beilstein J. Org. Chem. 2012, 8, 112–117, doi:10.3762/bjoc.8.12

- involved in electrostatic halogen bonding. Supporting Information Supporting Information File 36: Optimized structures for all minima of 2'-haloflavonols and the corresponding Cartesian coordinates. Acknowledgements The authors thank FAPEMIG and FAPESP for the financial support of this research, as well
Supramolecular chemistry II
Beilstein J. Org. Chem. 2011, 7, 1541–1542, doi:10.3762/bjoc.7.181

- synthetic receptors, crystallographic studies of halogen bonding and the use of polymers for protein binding. The second series again has a broad scope, as you will discover in the coming months as the series develops. With the second Thematic Series on supramolecular chemistry, we wish to contribute to the
The subtle balance of weak supramolecular interactions: The hierarchy of halogen and hydrogen bonds in haloanilinium and halopyridinium salts
Beilstein J. Org. Chem. 2010, 6, No. 4, doi:10.3762/bjoc.6.4
- -ClPhNH3H2PO4 (8), 3-IPyBnCl (9), 3-IPyHCl (10) and 3-IPyH-5NIPA (3-iodopyridinium 5-nitroisophthalate, 13), where hydrogen or/and halogen bonding represents the most relevant non-covalent interactions, has been prepared and characterized by single crystal X-ray diffraction. This series was further complemented
- it was possible to highlight the balance between non-covalent forces acting in these systems, where the relative strength of the halogen bonding C–X···A− (X = I, Br or Cl) and the ratio between the halogen and hydrogen bonds [C–X···A− : D–H···A−] varied across the series. Keywords: crystal
- engineering; halogen bonding; hydrogen bonding; supramolecular chemistry; weak interactions; Introduction Non-covalent interaction, such as hydrogen bonding and metal coordination represent the basic set of tools for the construction of elaborate architectures in the supramolecular chemistry of organic or






































































































































































































































































































































![[Graphic 1]](/bjoc/content/inline/1860-5397-11-222-i19.png?max-width=637&scale=1.18182) 16 and dynamic [2]catenane formed by compounds 17–19.
16 and dynamic [2]catenane formed by compounds 17–19.







































